This website contains detailed medical information designed to support the DESTINY Breast-12 study. The information is intended to be used by healthcare professionals involved in this study only and not for any other reason
0
target met for number of patients dosed in
Destiny Breast-12!
Key objectives of DESTINY BREAST-12 are to determine:
- the overall treatment effect of T-DXd in HER2-positive metastatic breast cancer (mBC) patients with or without baseline brain metastases (BM).
- the treatment effect on the development and progression of BM in patients with or without baseline BM using additional efficacy measurements.
- T-DXd’s efficacy in patients with stable or untreated BM.
- the effect of T-DXd on symptoms, functioning and HRQoL in HER2-postive mBC patients with or without baseline BM.
- the safety profile of T-DXd.
What is being investigated?
T-DXd is an antibody-drug conjugate that targets HER2; it is currently approved in the US, Europe and Japan. Participants will receive intravenous T-DXd 5.4 mg/kg every 3 weeks (21-day cycle) for as long as they continue to show clinical benefit (determined by the study investigator).
Who is participating in this study?
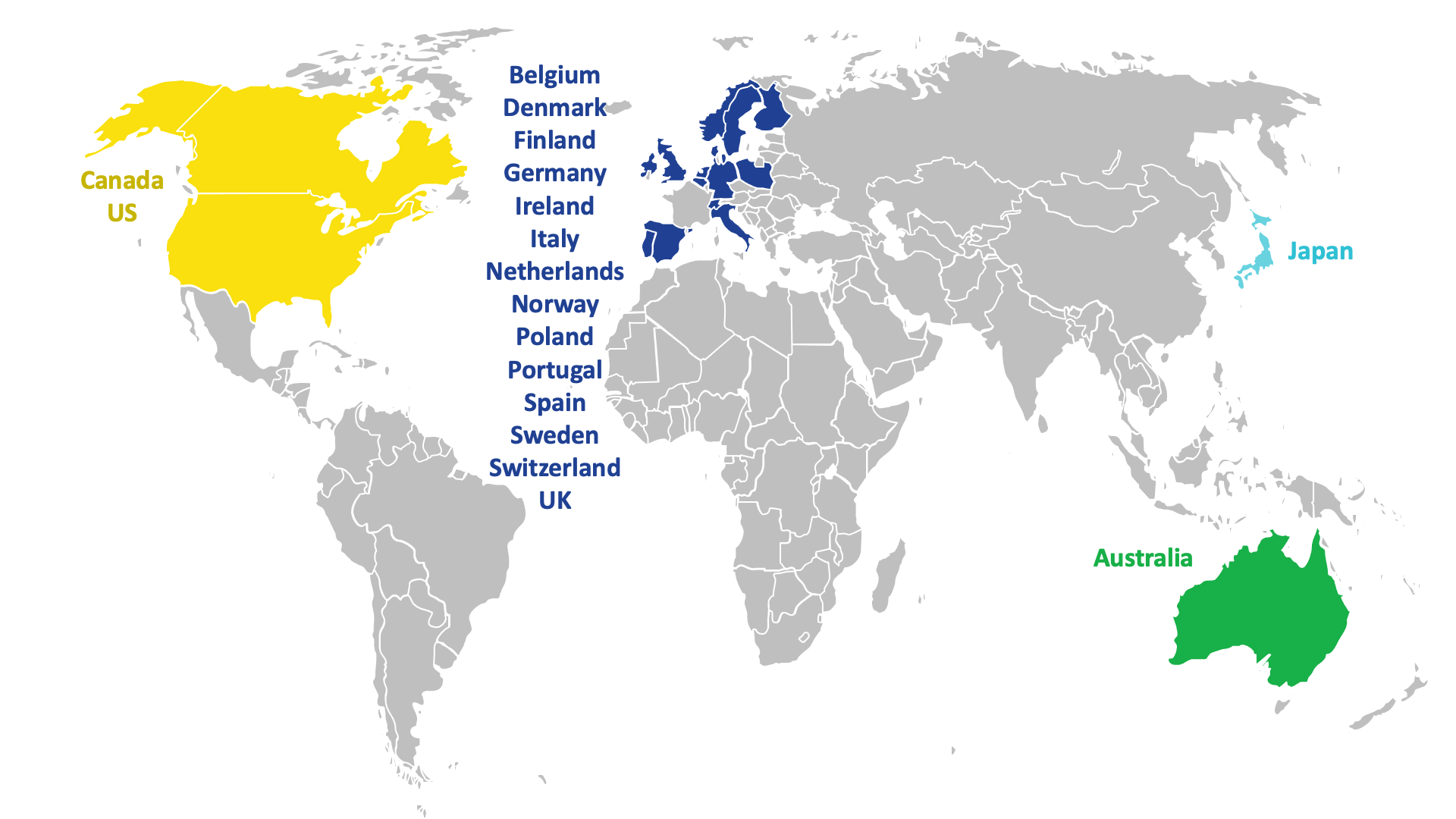
Around 500 patients in 19 countries across the world are expected to take part in DESTINY BREAST-12.
Eligible participants must:
- be ≥ 18 years of age*.
- have a diagnosis of unresectable/advanced or metastatic breast cancer and a confirmed HER2-positive expression.
- have radiologic or objective evidence of disease progression on trastuzumab, pertuzumab, or T-DM1 (≤ 2 lines/regimens of therapy in the metastatic setting).
*For participants aged < 20 years and enrolled in Japan, a written informed consent should be obtained from the participant and his or her legally acceptable representative.
References
1. Pestalozzi BC, Holmes E, de Azambuja E, et al. Lancet Oncol. 2013;14(3):244–248.
By selecting the button below you acknowledge you are leaving the Destiny Breast-12 Study website and continuing to another website not covered under AstraZeneca's website Privacy Policy.
Proceed to site